Product code: Fda number 2024 for importers
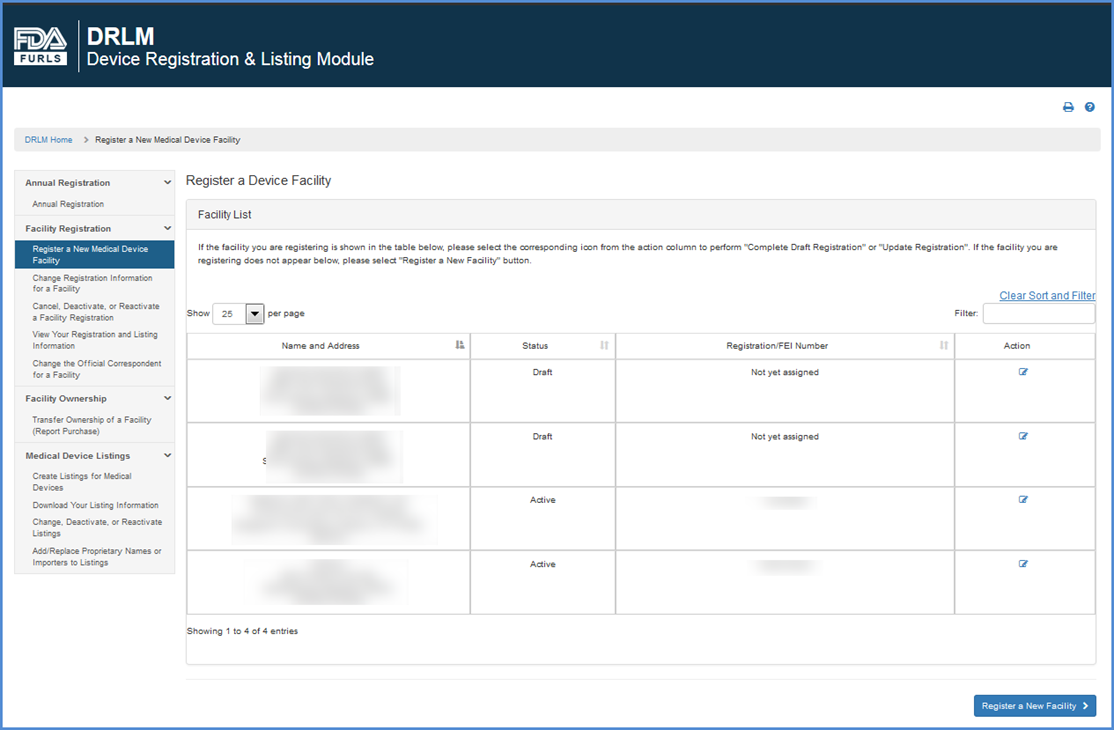
FDA Webinar on Human Drug Importations Customs International 2024, Add Replace Proprietary Names or Importers to Listings 2024, Import Basics FDA 2024, FDA Warning Reminder FSVP Importers Require DUNS Number as of 2024, FDA Registration 2024, What are the FDA requirements for food USA Food regulations 2024, Contact the FDA Import Program FDA 2024, Foreign Existing Reg in Account 2024, Register a New Medical Device Facility Step by Step Instructions 2024, Importing FDA Regulated Products The Import Process YouTube 2024, FDA Import Alerts How to Get Off a Red List 2024, Import Offices and Ports of Entry FDA 2024, Home FDAImports 2024, FDA Import Process FDA 2024, US FDA Registration is required for your product Understanding 2024, How to Successfully Import Natural Products to the USA PPT 2024, Import Alerts Customs International Trade Law Firm 2024, Register a New Medical Device Facility Step by Step Instructions 2024, Importing FDA Regulated Products Human Foods 2024, PPT US FDA Prior Notice PowerPoint Presentation free download 2024, FDA VQIP Applications for 2024 Opening Soon to Food Importers 2024, Importing Medical Devices 5 Key Factors to Ensure Regulatory 2024, Customs Broker Los Angeles FDA Prior Notice Filing 2024, Number of US FDA import detentions of Indian spice and spice 2024, Food Licenses FSSAI FDA Fire And Trade Import Export Licenses 2024, FDA Registration Product Announcement FDA PMA Product Notice 2024, FDA Recognizes DUNS Number as Acceptable Identifier for Importers 2024, US FDA Registration Consultants For India and Other Destinations 2024, How to Successfully Import Natural Products to the USA PPT 2024, Do Food Importers Need to Register with FDA Registrar Corp 2024, The U.S. Food and Drug Administration FDA Import Requirements 2024, FDA import refusals for adulteration or misbranding violations 2024, The procedure for Import Drug SFDA Registration China CFDA SFDA 2024, A Practical Guide to Importing Food and Ingredients to the U.S 2024, 13 334 made in India items rejected by US FDA in 6 yrs 1 753 2024.
FDA Webinar on Human Drug Importations Customs International 2024, Add Replace Proprietary Names or Importers to Listings 2024, Import Basics FDA 2024, FDA Warning Reminder FSVP Importers Require DUNS Number as of 2024, FDA Registration 2024, What are the FDA requirements for food USA Food regulations 2024, Contact the FDA Import Program FDA 2024, Foreign Existing Reg in Account 2024, Register a New Medical Device Facility Step by Step Instructions 2024, Importing FDA Regulated Products The Import Process YouTube 2024, FDA Import Alerts How to Get Off a Red List 2024, Import Offices and Ports of Entry FDA 2024, Home FDAImports 2024, FDA Import Process FDA 2024, US FDA Registration is required for your product Understanding 2024, How to Successfully Import Natural Products to the USA PPT 2024, Import Alerts Customs International Trade Law Firm 2024, Register a New Medical Device Facility Step by Step Instructions 2024, Importing FDA Regulated Products Human Foods 2024, PPT US FDA Prior Notice PowerPoint Presentation free download 2024, FDA VQIP Applications for 2024 Opening Soon to Food Importers 2024, Importing Medical Devices 5 Key Factors to Ensure Regulatory 2024, Customs Broker Los Angeles FDA Prior Notice Filing 2024, Number of US FDA import detentions of Indian spice and spice 2024, Food Licenses FSSAI FDA Fire And Trade Import Export Licenses 2024, FDA Registration Product Announcement FDA PMA Product Notice 2024, FDA Recognizes DUNS Number as Acceptable Identifier for Importers 2024, US FDA Registration Consultants For India and Other Destinations 2024, How to Successfully Import Natural Products to the USA PPT 2024, Do Food Importers Need to Register with FDA Registrar Corp 2024, The U.S. Food and Drug Administration FDA Import Requirements 2024, FDA import refusals for adulteration or misbranding violations 2024, The procedure for Import Drug SFDA Registration China CFDA SFDA 2024, A Practical Guide to Importing Food and Ingredients to the U.S 2024, 13 334 made in India items rejected by US FDA in 6 yrs 1 753 2024.